Hot Melt Extrusion
Hot melt extrusion (HME) is the process of applying heat and pressure to melt a polymer and forcing it through an orifice in a continuous process. HME is well-known, developed to produce polymer products of uniform shape and density, and its industrial application dates back to the 1930s1. It is one of the most widely applied processing technologies in the plastic, rubber, and food industries and is used to prepare more than half of all plastic products including bags, films, sheets, tubes, fibers, foams, and pipes2. HME has more recently been applied to the healthcare industry where it is used to manufacture medical devices and mix active pharmaceutical ingredients (APIs) with polymers3. HME is used to enhance the API’s bioavailability or prepare precursors for thermoplastic drug-eluting devices, such as subcutaneous and intraocular implants and intravaginal rings. This technical brief discusses the equipment and principles of HME with an emphasis on its use in the pharmaceutical industry.
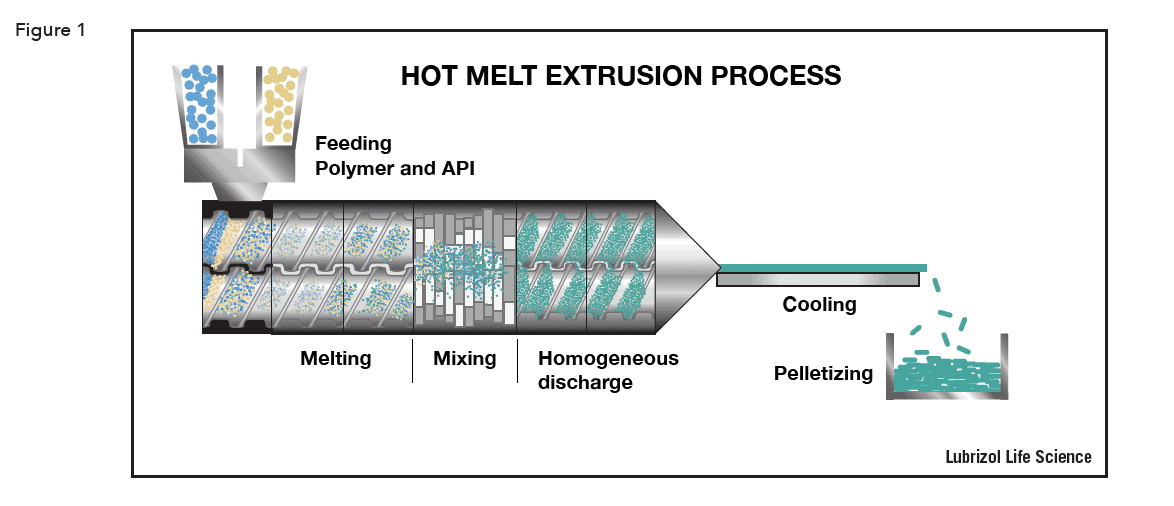 HME is carried out using an extruder – a barrel containing one or two rotating screws that transport material down the barrel. Extruders consist of four distinct parts:
HME is carried out using an extruder – a barrel containing one or two rotating screws that transport material down the barrel. Extruders consist of four distinct parts:
- An opening though which material enters the barrel, which may have a hopper filled with the material(s) to be extruded or be continuously supplied in a controlled manner by one or more external feeder(s).
- A conveying (process) section, which comprises the barrel and the screw(s) that transport and, where applicable, mix the ma
- An orifice (die) for shaping the material as it leaves the
- Downstream auxiliary equipment for cooling, cutting, and/or collecting the finished
The HME process is shown schematically in Figure 1.
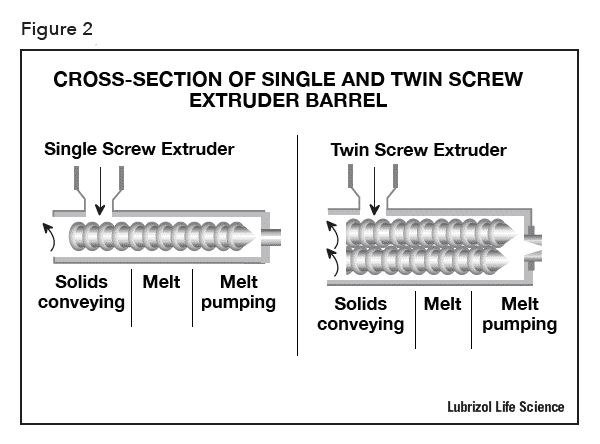
There are two types of extruders: single and twin screw extruders (see Figure 2). Single screw extruders are primarily used for melting and conveying polymers to extrude them into continuous shapes whereas twin screw extruders are used for melt-mixing polymers with additional materials (pigments, fillers, reinforcers, and APIs), and for devolatilization. In the production of pharmaceutical formulations, which require homogeneous and consistent mixing of multiple formulation ingredients, a twin screw extruder is preferred because the rotation of the inter-meshing screws provides better mixing to produce a homogeneous solid containing finely dispersed API particles or a solid-solution of API in polymer. Consistent melt-mixing via twin screw extrusion can improve the dissolution rate and bioavailability of poorly water-soluble API formulations. Uniformly distributed API is also a prerequisite to produce drug-eluting devices with intra- and inter-batch reproducibility of drug-release kinetics.
 Melting is accomplished by frictional heating within the barrel. For twin-screw extruders, the materials undergo shearing between the rotating screws and between the screws and the wall of the barrel as they are conveyed. The barrel can be heated with barrel-mounted heaters on the or cooled with water. The barrel section temperatures are usually optimized so that the material viscosity is low enough to allow proper mixing and conveyance down the barrel, while also keeping temperatures low enough to avoid thermal degradation.
Melting is accomplished by frictional heating within the barrel. For twin-screw extruders, the materials undergo shearing between the rotating screws and between the screws and the wall of the barrel as they are conveyed. The barrel can be heated with barrel-mounted heaters on the or cooled with water. The barrel section temperatures are usually optimized so that the material viscosity is low enough to allow proper mixing and conveyance down the barrel, while also keeping temperatures low enough to avoid thermal degradation.
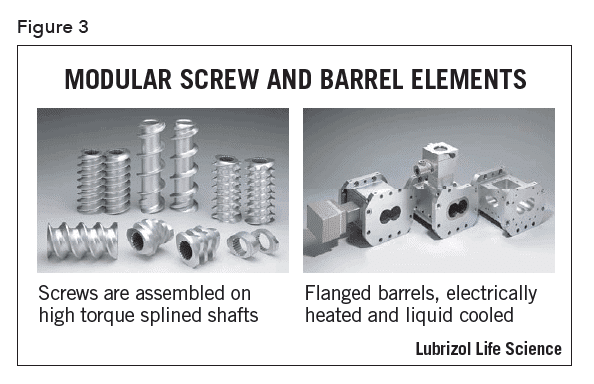
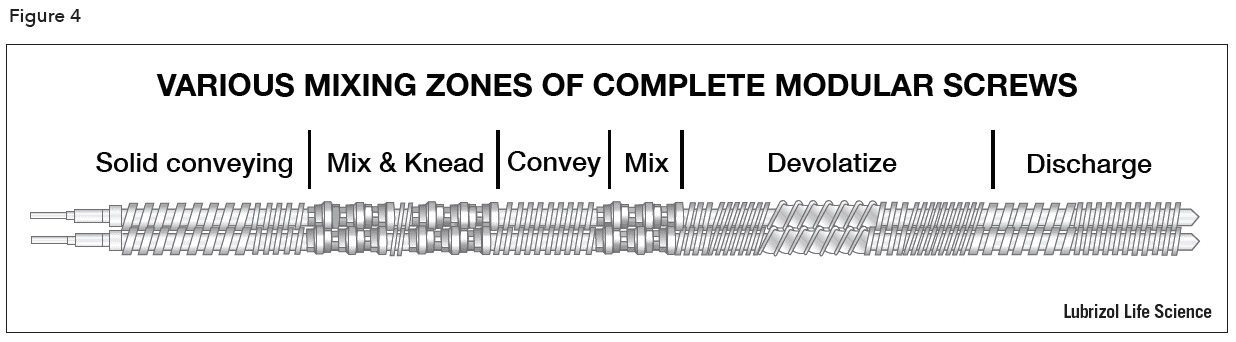
The screws of a twin screw extruder are usually engineered to provide different types of mixing and conveying conditions at various zones in the barrel. During product development, modular screws with multiple elements (Figure 3) fitted on a common shaft allow the tailoring and optimization of the screw design for each product. Sections of the screw can be designed to perform particle size reduction, mixing, and conveying functions. The length of the screw in relation to the barrel diameter (the L/D ratio) is chosen to optimize the degree of mixing and the number of zones required to achieve the final product characteristics. An example of a complete modular screw is show in Figure 4. Single piece production screws may be built to the same design as the development screws and are easier to clean for cGMP compliance.
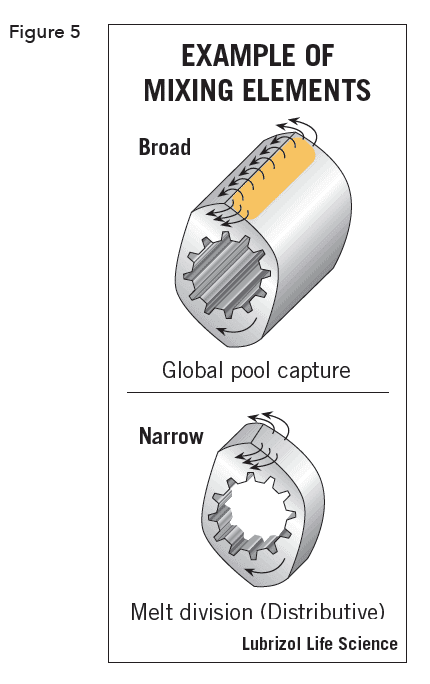
 Rotation of the screws creates distributive and dispersive mixing (Figure 5). Distributive mixing maximizes the division and recombination of the materials while minimizing energy input by mixing with low extensional and planar-shear effects. This uniformly blends the materials but does not significantly reduce dispersed material particle size and yields minimal thermal and shear degradation of sensitive materials.
Rotation of the screws creates distributive and dispersive mixing (Figure 5). Distributive mixing maximizes the division and recombination of the materials while minimizing energy input by mixing with low extensional and planar-shear effects. This uniformly blends the materials but does not significantly reduce dispersed material particle size and yields minimal thermal and shear degradation of sensitive materials.
 Dispersive mixing applies extensional and planar shear fields to break the dispersed materials to smaller size, ideally using energy at or slightly above the threshold level needed to break them down.
Dispersive mixing applies extensional and planar shear fields to break the dispersed materials to smaller size, ideally using energy at or slightly above the threshold level needed to break them down.
The use of different mixing elements allows the twin screw extruder to perform both particle size reduction and mixing so that the APIs can be incorporated into the polymer in dispersed form or, if the API solubility in the polymer is high enough, in dissolved form. Since the extrudate cools rapidly on exiting the extruder, any API that is dissolved in the polymer at the mixing temperature may be unable to recrystallize on cooling, leading to supersaturated solid solutions. In such cases, stability of the product must be closely followed as recrystallization of the API over long time-scales is possible, especially at elevated storage temperatures and high API loadings, which may impact the shelf life of the final product.
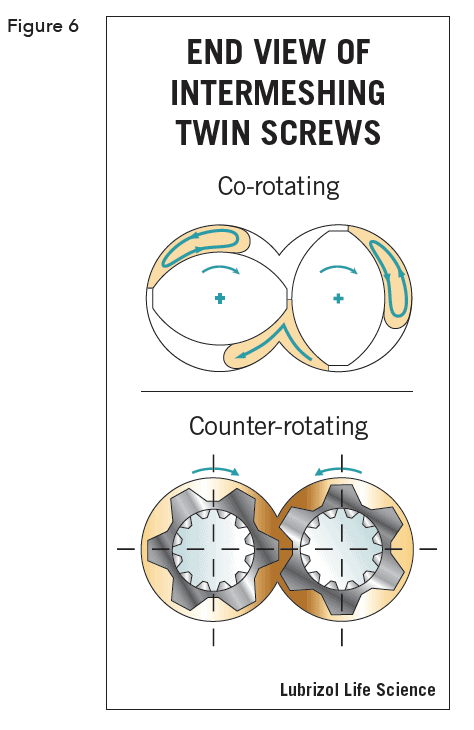
There are two families of twin screw extruders: high-speed energy input (HSEI) twin-screw extruders, which are primarily used for compounding, reactive processing, and/or devolatilization, and low-speed late fusion (LSLF) twin-screw extruders, which are designed to mix at low shear and pump at uniform pressures. Screws may be co-rotating (self-wiping), which is the most popular, or counter- rotating (calendar gap). See Figure 6.
 Different types of exit dies are used to shape the extrudate to the desired profile. These dies include sheet and film dies used in transdermal film applications, strand dies used for medical tubing and some drug-eluting devices, shape dies used in blow molding, and co- extrusion dies used in reservoir device designs. Different downstream auxiliary components are also used in the finishing process, including water baths and air knives for cooling, conveyor belts for moving the extruded product from the die to the end of the line, strand-cutters for cutting the extrudate into tubing or rods, and spoolers for extrudate collection. Pelletizers are used for cutting the extrudate into smaller pieces for direct capsule filling and, in the case of some devices, for injection molding to form the final product.
Different types of exit dies are used to shape the extrudate to the desired profile. These dies include sheet and film dies used in transdermal film applications, strand dies used for medical tubing and some drug-eluting devices, shape dies used in blow molding, and co- extrusion dies used in reservoir device designs. Different downstream auxiliary components are also used in the finishing process, including water baths and air knives for cooling, conveyor belts for moving the extruded product from the die to the end of the line, strand-cutters for cutting the extrudate into tubing or rods, and spoolers for extrudate collection. Pelletizers are used for cutting the extrudate into smaller pieces for direct capsule filling and, in the case of some devices, for injection molding to form the final product.
As with any dosage form, material selection is critical in the development of a successful product. For most applications, the polymer should be thermoplastic, stable at the temperatures used in the process, and chemically compatible with the API during extrusion. For solid oral dosage forms, water soluble polymers are usually chosen from among well-known polymers already used in pharmaceutical products such as poly(ethylene glycol) and poly(vinylpyrrolidinone). With the increased interest in using HME for pharmaceutical products, major polymer suppliers are also beginning to offer polymers specifically designed for pharmaceutical applications. For drug-eluting devices, the polymers are generally water-insoluble, with most products under development using either ethylene vinyl acetate copolymers (EVAs) or polyurethanes.
HME allows the API to be mixed with the polymer under the minimum of shear and thermal stresses and, hence, with the formation of minimal process-related API degradants. Antioxidants are often included within the formulation, and the short residence time in the barrel (typically on the order of minutes) also helps to minimize thermal degradation, especially compared to batch mixing and other compounding processes.
One strategy for controlling drug elution kinetics from devices such as intravaginal rings involves an extension of the simple extrusion technique. Simultaneous extrusion of a drug-loaded core strand with a release-controlling polymer sheath that encapsulates the core in a single co-extrusion process produces a two-layer core-sheath strand. A specially designed extrusion head is fed by two perpendicular extruders – one supplying the core composition and the other supplying the sheath material. The core-sheath strand is cut and the ends connected to make the final device.
HME provides product developers of medical devices, dissolving oral dosage forms, and drug-eluting devices with a processing option that maximizes API mixing with polymer while minimizing API degradation and even opens the door to products that cannot be prepared by other means.
References
- Rauwendaal Polymer Extrusion, Hanser Publishers, München (1986) 20-25.
- Kruder Extrusion. In: Encyclopedia of Polymer Science and Engineering Vol. 1, 2nd ed. John Wiley & Sons Inc., New York (1985) 571-631.
- Crowley MM, Zhang F, Repka MA, Thumma S, Upadhye SB, Battu SK, McGinity JW, Martin Pharmaceutical Applications of Hot Melt Extrusion: Part 1
