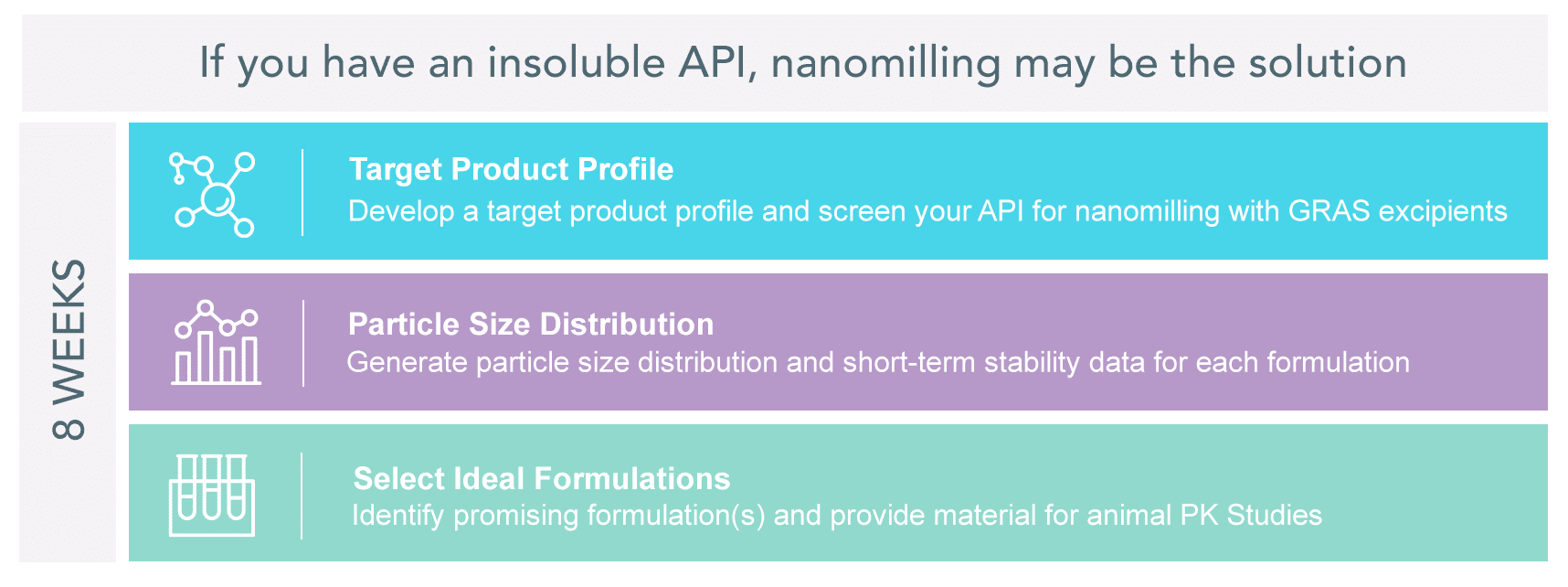
Drug Nanomilling Feasibility Services for Water Insoluble APIs
As many as 90% of active pharmaceutical ingredients (APIs) in the discovery pipeline are poorly water-soluble, resulting in poor bioavailability or prohibiting injectable dosage form development. Particle Sciences uses a host of techniques to increase the bioavailability of APIs, including inclusion complexation, API chemical modification, and API physical modification. While there is no one-size-fits-all all, we have identified nanomilling as a particularly efficient, reproducible, and scalable process to increase API solubility and formulate poorly water-soluble APIs.

Why partner with Particle Sciences?
Nanomilling is an efficient, reproducible, and scalable approach for improving the rate of dissolution and bioavailability of BCS Class II and IV compounds.
Particle Sciences has decades of experience developing nanoparticulate suspensions with commercial milling equipment and our own proprietary mills. We are the only CDMO capable of performing nanomilling under aseptic conditions. If you choose to proceed with your nanomilling project, we have the personnel, facilities, and equipment to produce clinical and commercial scale nanomilled formulations. If you have an insoluble API, contact us below to learn how nanomilling can help to solve your formulation challenges, and let us take your product from concept to commercial.
- Pharmaceutically elegant formulations: No harsh organic solvents or pH extremes; most nanomilled suspensions are aqueous-based and it may be possible to achieve high API concentrations (5 – 40+% (w/w))
- Easy scale-up: Commercial nanomilling equipment utilizes a recirculation process that allows batch sizes to increase without changing process variables
- Reproducibility: Once a nanomilling process is optimized, there is minimal variation in particle size distribution from batch-to-batch
Frequently Asked Questions
What is the basis for nanomilling increasing oral bioavailability?
As the particle size of the API is reduced, the surface area increases (as an inverse function of particle diameter). This results in an increase in the rate of dissolution and may result in dramatic increases in bioavailability and/or significant reductions in food effects (i.e., the variability in fed versus fasted drug uptake).
Is nanomilling technology commercially validated?
Yes, as of September 2022, there were 18 marketed drug products that utilize nanomilling as the enabling drug delivery technology.
What are the advantages of nanomilling for parenterally administered APIs?
You may be able to develop a pharmaceutically elegant formulation, I.e., aqueous based, no harsh organic solvents, buffered, isotonic, and formulated at physiologic pH (specific formulation attributes are a function of the API, process and formulation).
If the nanomilling feasibility screen produces a good preclinical outcome, how far into development can Particle Sciences take my API?
We are able to optimize the formulation into a clinical-ready dosage form, scale up the nanomilling process, manufacture GLP test articles, identify the sterilization method (for parenteral, otic or ophthalmic routes of administration), and progress the program into cGMP manufacturing. We are also able to conduct ICH-compliant stability programs to support regulatory submissions. Depending on the specific API and commercial volumes, we may be able to take the product into commercial manufacture.
