A 2023 Guide to Vaccine Development & Immunization
Infectious diseases pose significant global health issues, and the introduction of prophylactic vaccination has been critical in combatting them. The COVID-19 pandemic, caused by the novel SARS-CoV-2 coronavirus, highlights the need for safe and effective vaccines against new pathogens. Dozens of companies and research groups are working on vaccines for COVID-19 as of April 20201.
This post highlights the historical toll of infectious diseases, uncovers the innate defenses of the immune system, and explains how a vaccine can trigger an adaptive immune response to provide protection against disease. We also explore how the efficacy and safety of a vaccine are dictated by its formulation and discuss vaccine formulation in the context of the five types of vaccines—highlighting those that require an adjuvant or are based on RNA (ribonucleic acid). In these cases, efficacy can vary by orders of magnitude as a result of exquisitely small changes in the formulation.
The History of Infectious Disease
Throughout modern history, humans have been battling infectious diseases. In the seventeenth century, one-third of Europe’s population died from a plague carried by the bacterium Yersinia pestis. In the following century, an estimated forty million people died from smallpox. By the nineteenth century, tuberculosis had killed one in seven of all people that had ever lived2. The twentieth century opened with the H1N1 ‘Spanish Flu’ pandemic, which infected one-third of the world’s population and killed fifty million3. It closed with the outbreak of the AIDS epidemic, caused by HIV, which has now infected seventy-five million and killed more than thirty million4. In the interim years, smallpox killed a staggering 300 million people5 before it was eradicated6.
The Role of Vaccination
Smallpox was the first, and so far, only, infectious disease that affects humans to be eradicated. The announcement was made in 1979 and came as the result of a global vaccination program, concluding two centuries of research. In 1768, Dr. John Fewster reduced the chance of his patients contracting smallpox by inoculating them with pus from the sores of an infected person7. Some died from the resulting smallpox infection. Those that survived it were immune to further infection. Thirty years later Dr. Edward Jenner discovered that immunity against smallpox could also be achieved using fluid from sores of someone infected with cowpox8. Cowpox infections rarely led to death, and Jenner’s safer approach marked the birth of vaccination – perhaps one of the biggest contributions to human and animal health globally.
Vaccination programs have limited polio, measles, and tetanus infections and are effective against influenza, HPV, and chickenpox. The World Health Organization (WHO) reports that licensed vaccines are currently available for twenty-five different preventable infections9.
The Immune System
Pathogens constantly try to invade our bodies. Without an immune system, every infection would probably lead to death. Evolution has given us two defense systems. The first, the innate immune system, comprises both the external barriers that keep pathogens out (shown in Figure 1) and a system of cells that secrete chemicals to kill invaders that manage to get in.
Figure 1: Physical barriers of the innate immune system
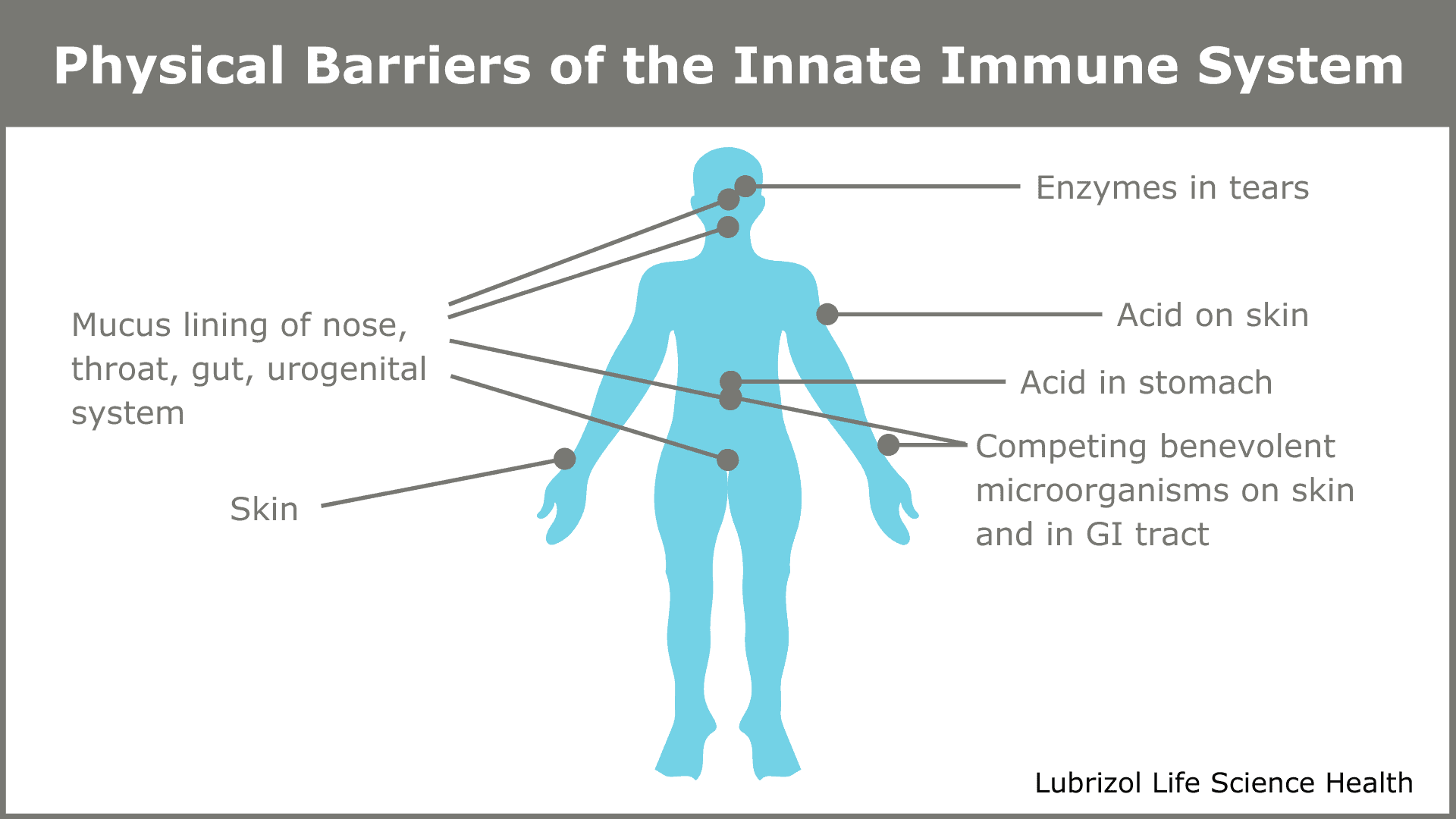
The innate system reacts rapidly to pathogens and provides a first line of defense, but it is not targeted at any particular pathogen. The innate system buys time while the second system—called the adaptive, or acquired, immune system—becomes active. The adaptive immune system is a marvel of nature—a slower, exquisitely choreographed dance of specialized cells that results in the destruction of invading pathogens and any host cells they managed to infect. Paradoxically, it may have been a retrovirus infection in one of our distant ancestors that provided the genetic code for the components of an adaptive immune system10.
B-Cells, T-Cells, and Immunity
The B-cell is the key player. There are approximately ten billion B-cells circulating in the human body, each carrying ten thousand copies of a unique antibody. If a B-cell bumps into a pathogen that its antibody binds to, that activated B-cell begins to produce new copies of its antibody at a rate of two thousand per second. The released antibodies bind to the pathogens, immobilizing them and marking them for destruction by other types of cells.
The activated B-cells go into overdrive and divide rapidly to produce daughter cells. Some daughter cells become additional antibody factories, and some migrate to the bone marrow where they can survive for decades. They harbor the memory of the pathogen and are ready to mount a fast-response attack if it is ever encountered again.
Simultaneously, T-cells recognize host cells that have been infected by the pathogen and destroy them. Activated T-cells also proliferate, forming a colony of ‘memory’ cells able to destroy host cells infected by that pathogen again in the future.
Vaccines leverage the adaptive immune system’s ability to remember. A vaccine contains a carefully chosen agent that stimulates the adaptive immune system to provide long-lasting immunity to a particular disease. More recently, the knowledge gained through prophylactic vaccination has led to the development of therapeutic vaccines against cancer11, and even treatments for drug abuse12.
Vaccine Types
The WHO groups vaccines into four types (see Table 1)13. Each vaccine group is defined according to the kind of agent employed to trigger the immune system to react.
Table 1: Types of vaccines with examples
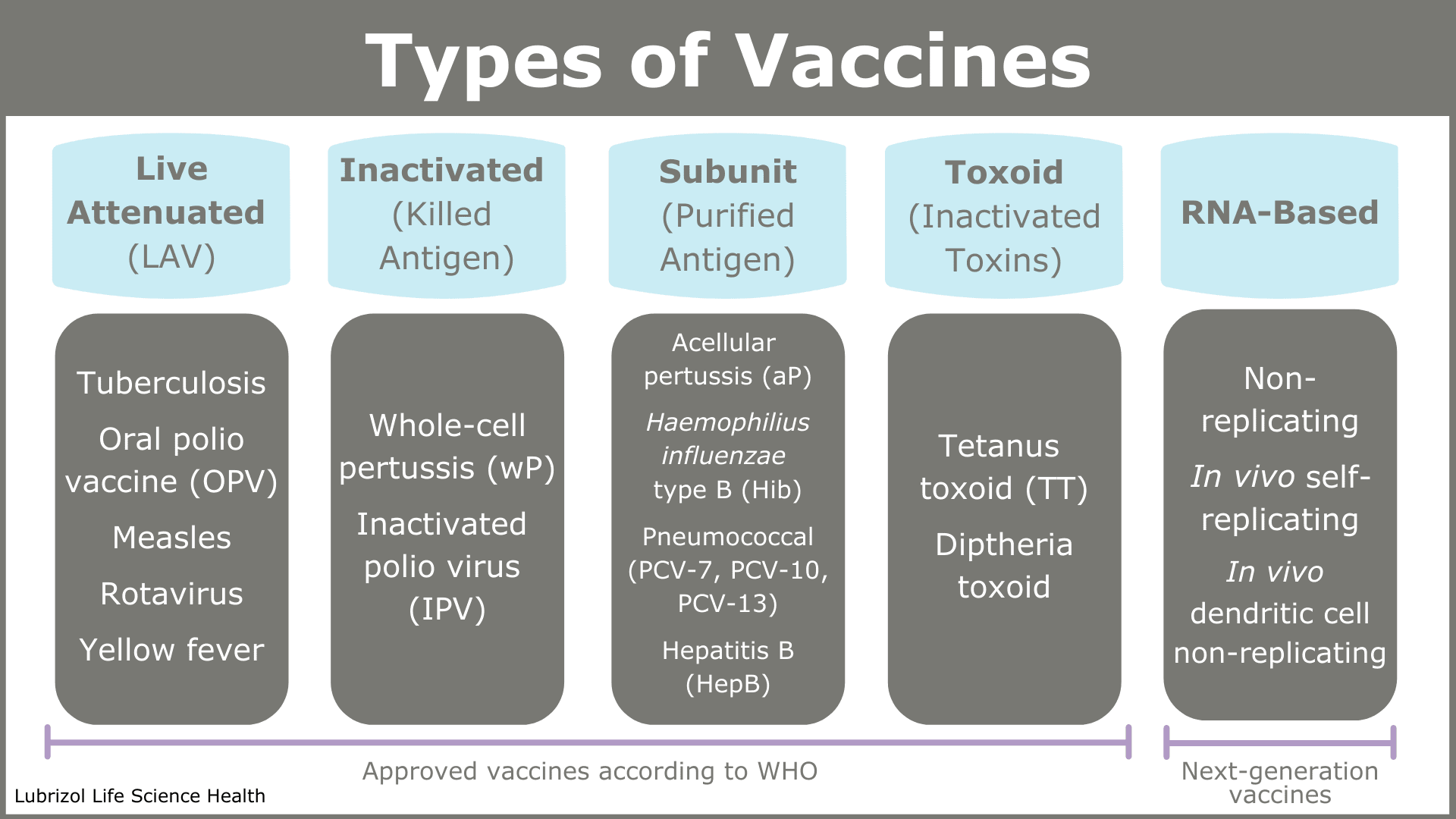
The active agent is called the antigen, and as Table 1 shows, antigens can be a whole pathogen, part of a pathogen, or a toxin produced by a pathogen.
Live Attenuated Virus (LAV) Vaccines
Live attenuated virus vaccines are based on viruses that have been weakened in a laboratory so that they do not proliferate well in a host. This allows the host time to generate an immune response in the absence of serious disease. Since attenuated viruses most closely resemble the wild pathogen, this type of vaccine stimulates a strong immune response and provides robust immunity.
Inactivated (Killed) Antigen Vaccines
Inactivated antigen vaccines contain killed bacteria or inactivated viruses that cannot proliferate. Since they contain all the chemical motifs of the live pathogen, they stimulate the adaptive immune system to react, but they are not as effective as live attenuated vaccines and require multiple doses. They are, however, safer and more stable products.
Subunit and Toxoid (Inactivated Toxin) Vaccines
Subunit and toxoid vaccines do not contain any microbes at all. The subunit antigen is a protein or a carbohydrate found on the surface of the pathogen. The immune system is therefore triggered to just recognize that one part of an invading pathogen. These vaccines often require multiple doses to be effective.
RNA-Based Vaccines
RNA-based vaccines are a relatively recent development, but they are the subject of significant research interest due to their accelerated development timelines relative to other vaccines. Scientists sequence the genome of a viral pathogen and determine which sections code for proteins that could make a good antigen. They then produce mRNA—genetic material that codes for that protein—purify it, and formulate it as a vaccine. On administration, the subject’s cellular machinery translates the mRNA into the protein antigen in vivo.
An RNA-based approach is being used by several of the groups developing COVID-19 vaccines. Initial clinical trials for the first COVID-19 mRNA vaccine candidate started just weeks after the SARS-Cov-2 virus was sequenced. Such short development times are unprecedented in vaccine development. As such, mRNA vaccines represent a particularly promising approach for vaccines against novel pathogens.
Sources of Antigen
Whole viral and bacterial antigens are grown in highly controlled lab cultures and require substantial purification. Traces of antibiotics used to prevent bacterial growth in the media while growing viruses may be found in the final vaccine.
Sub-unit protein antigens are usually derived from yeast cells that have been genetically modified to produce the protein during incubation in cell culture. Significant processing is required to isolate a highly purified antigen.
RNA is produced in a lab by advanced chemical synthesis techniques and often encapsulated in lipidic or polymeric particles to protect the molecule within the body.
The Importance of Formulation
Vaccines are sterile mixtures of several components, and proper formulation is fundamental to a vaccine’s success or failure. Apart from the antigen, which was discussed above, the basic components of a vaccine may include stabilizers, preservatives, adjuvants, and carrier particles14.
Stabilizers for Vaccines
Vaccine stability is key to efficacy. Stabilizers such as MgCl2 for oral polio vaccine (OPV), MgSO4 for measles, lactose-sorbitol for chickenpox, and sorbitol-gelatin for measles, mumps, and rubella are used to protect the delicate antigen from degradation during storage. This is especially important if the chain of cold storage cannot be guaranteed during shipping of a vaccine. Correct choice of stabilizer depends on the antigen, and omitted or improperly chosen stabilizers can lead to vaccines that lose their potency and become ineffective.
Preservatives for Vaccines
For vaccines in multidose containers, a preservative may be required to prevent microbial growth. Thiomersal, formaldehyde, or phenol derivatives are most common.
Adjuvants for Vaccines
Inactivated antigen vaccines and sub-unit vaccines generate a relatively weak immune response and require properly formulated ‘helpers’ called adjuvants to provide robust and long-lasting immunity. The most common adjuvant used in human vaccines is alum, which comprises particles of insoluble aluminum salts. However, there are several other commercial examples of adjuvants:
- Nanodroplets of squalene oil emulsified in water using non-ionic emulsifiers provide the basis of Novartis’ MF59® adjuvant formulation.
- GSK developed a number of proprietary nanoparticle-based adjuvant systems, all designated by the prefix ‘AS’.
- Novavax uses a proprietary lipid adjuvant formulation.
- Advanced BioAdjuvants developed an adjuvant based on a mixture of polyacrylic acid and phospholipids.
Vaccine Manufacturing Considerations
Since most vaccines are designed for administration via needle, they are manufactured as sterile products. Furthermore, vaccines that are formulated as nano-emulsions require high-pressure homogenization equipment, and RNA vaccines require specialized manufacturing equipment that combines the RNA with the lipids to form nanoparticles under highly defined mixing conditions in microfluidic channels.
Since all vaccines are multiphase particulate systems, sterile filtration is often not possible. Additionally, because vaccines contain fragile biomolecules, they cannot be steam-sterilized or irradiated with e-beams or gamma rays. This leaves aseptic manufacturing using sterile starting materials as the only viable option in most cases.
Aseptic manufacturing is widely recognized as one of the most difficult challenges in the pharmaceutical industry and must be conducted in an appropriate facility with the proper quality assurance (QA) oversight. Operations must be conducted by properly trained staff who appreciate the complexity and delicacy of the product as vaccine manufacture is far from a simple fill-finish operation.
Conclusion
Vaccines and vaccination have saved countless millions of lives and changed the face of public health. The development of new products requires an understanding of the pathogen, the immune system, and a deep understanding of vaccine formulation principles—from adjuvants to finished product.
Particle Sciences is a trusted development and manufacturing partner with experience formulating vaccines, and our SATx technology is a proven carrier for biologics. If you are looking for a CDMO to help develop your vaccine product or scale-up a manufacturing process in a GMP environment, turn to the knowledgeable, client-focused team at Particle Sciences.
References
- COVID-19 vaccine tracker | RAPS. https://www.raps.org/news-and-articles/news-articles/2020/3/covid-19-vaccine-tracker.
- Murray JF. Mycobacterium tuberculosis and the cause of consumption: From discovery to fact. American Journal of Respiratory and Critical Care Medicine. 2004;169(10):1086-1088. doi:10.1164/rccm.200312-1639OE
- 1918 Pandemic (H1N1 virus) | Pandemic Influenza (Flu) | CDC. https://www.cdc.gov/flu/pandemic-resources/1918-pandemic-h1n1.html.
- WHO | HIV/AIDS. WHO. 2020.
- Rosengard AM. Smallpox: the fight to eradicate a global scourge. Journal of Clinical Investigation. 2003;112(12):1775-1775. doi:10.1172/jci20529
- WHO commemorates the 40th anniversary of smallpox eradication. https://www.who.int/news-room/detail/13-12-2019-who-commemorates-the-40th-anniversary-of-smallpox-eradication.
- The Milkmaid Who Supposedly Inspired The Smallpox Vaccine Was A Myth : Goats and Soda : NPR. https://www.npr.org/sections/goatsandsoda/2018/02/01/582370199/whats-the-real-story-about-the-milkmaid-and-the-smallpox-vaccine?t=1586174086203.
- Riedel S. Edward Jenner and the History of Smallpox and Vaccination. Baylor University Medical Center Proceedings. 2005;18(1):21-25. doi:10.1080/08998280.2005.11928028
- WHO | Global Vaccine Action Plan 2011-2020. WHO. 2017.
- Janeway J. How the immune system works to protect the host from infection: A personal view. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(13):7461-7468. doi:10.1073/pnas.131202998
- Immunotherapy. https://www.cancer.org/treatment/treatments-and-side-effects/treatment-types/immunotherapy.html.
- Vaccines against addictive drugs push forward despite past failures | February 19, 2018 Issue – Vol. 96 Issue 8 | Chemical & Engineering News. https://cen.acs.org/articles/96/i8/Vaccines-against-addictive-drugs-push.html.
- MODULE 2 – Types of vaccine – WHO Vaccine Safety Basics. https://vaccine-safety-training.org/types-of-vaccine.html.
- MODULE 2 – Components of a vaccine – WHO Vaccine Safety Basics. https://vaccine-safety-training.org/vaccine-components.html.
- Mucosal Vaccines – Aptimmune. http://aptimmune.com/mucosal/.
- Cullis PR, Hope MJ. Lipid Nanoparticle Systems for Enabling Gene Therapies. Molecular Therapy. 2017;25(7):1467-1475. doi:10.1016/j.ymthe.2017.03.013
Authors:

