Nasal Drug Formulation: A Guide to Successful Drug Product Development
With the rise of innovative biotherapeutics, novel delivery mechanisms are required to meet unresolved clinical needs. Nasal drug delivery offers an important alternative to injectables and orally ingested drugs for cases where local or systemic administration is desirable.
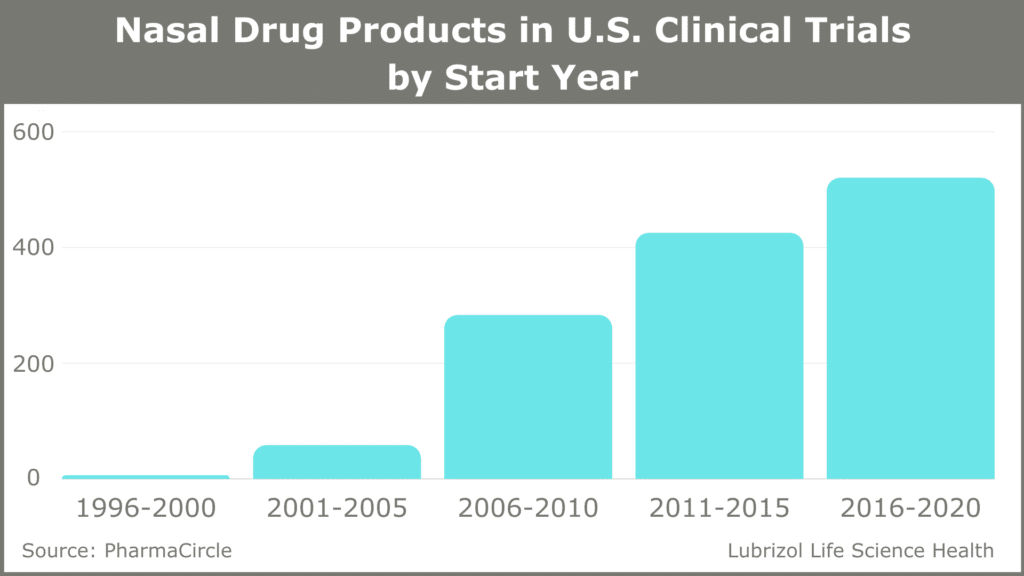
While liquid-based and dry powder nasal drugs make up a small fraction of all new drug approvals, they are an emerging class of medications with significant benefits. The inhalation and nasal spray generic drugs market was valued at $7.8 billion in 2018 and is predicted to reach close to $12 billion by the end of 2025.1 Most approved nasal drugs are liquid-based sprays and include small molecule drugs like sumatriptan for the treatment of migraines, as well as large molecules, including vitamin B12 and vasopressin. This range of applications has led to significant growth in the number of nasal drug products in U.S. clinical trials (see Figure 1).
Figure 1: Nasal Drug Products in U.S. Clinical Trials (1996-2020)
Here we look at the advantages of nasal drug delivery and the factors that go into the successful development and launch of nasal formulations of these complex drug products.
The Benefits of Administering Drugs to the Nasal Cavity
The nasal cavity is a key target for administering drugs locally, systemically, and even to the central nervous system (CNS). The olfactory region is a small portion of the surface area of the nasal cavity, making up only 5 cm2 of 160 cm2 total area. Liquid or particles that land on the nasal mucosa can either act locally or be absorbed into the bloodstream due to the extensive vascularization of this tissue. A wide range of drugs—corticosteroids, antihistamines, and others—can be delivered to the nasal cavity to treat localized conditions, such as congestion, sinusitis, and allergic reactions. Importantly, there are six nerves branching into the nasal cavity, including the first cranial nerve, or olfactory nerve, which is the only site at which the CNS is exposed to the body’s mucosa. This means drugs can be directly absorbed into the brain via this route, bypassing the blood-brain barrier. The table below shows top indications treated with nasally administered drug products as well as the APIs that are delivered via the nasal route.
Table 1: Top Indications and APIs Amenable to Nasal Drug Delivery
| Top Indications/Conditions | Top APIs |
| Influenza | Oxytocin |
| Rhinitis | Dexmedetomidine |
| Schizophrenia | Esketamine |
| Pain | Saline |
| Asthma | Midazolam |
| Sinusitis | Ketamine |
| Nasal Polyps | Budesonide |
| Autism Spectrum Disorder | Influenza Vaccines |
| Anesthesia | Fentanyl |
| COVID-19 | Fluticasone Propionate |
| Obesity | Insulin |
Nasal drugs have some limitations, which include the potential for rapid clearance from the nasal cavity, low bioavailability, challenges getting a large enough dose due to the small absorption area, and the risk to liquid-based sprays of microbial contamination.
Despite these potential limitations, the nasal administration of medications offers many benefits:
- It is non-invasive and easy to use compared to injectables, leading to improved patient acceptance and compliance.
- Absorption occurs directly into the bloodstream and bypasses the liver and first-pass metabolism, which can be an important consideration for drugs that are degraded in the GI tract.
- Rapid absorption leads to fast-acting systemic effects, which can be important for emergencies, even when a patient is unconscious. Examples include naloxone (opioid overdose), sumatriptan (migraines), Baqsimi® (glucagon for diabetics), and diazepam (epilepsy).2
- Delivery to the olfactory region of the nasal cavity allows direct access to the CNS for some neurological medications.
- It offers a method to administer diverse APIs for both local and systemic applications.
Case Study
Applications for Vaccine Delivery
Nasal delivery can also be a non-invasive method to administer vaccines. Nasal vaccines don’t rely on injections, are painless, and may be better at eliciting an immune response for some pathogens, like coronaviruses, that infect people via the nasal mucosa. Eliciting a mucosal immune response, including the recruitment of localized T cells and B cells that make IgA antibodies, may elicit a more effective immune response than injected vaccines for some infections. This response is part of the phenomenon known as sterilizing immunity, during which a pathogen is cleared from the body before it infects cells.
If you are interested in learning more about vaccine development and immunization, read our blog post on the subject.
Key Considerations for Successful Formulation Development
Nasal spray formulations may be water-based, hydroalcoholic, nonaqueous, suspensions, or emulsions. A formulation can include diverse excipients, including solvents, mucoadhesive agents, buffers, antioxidants, preservatives, and penetration enhancers (i.e., compounds to improve absorption or penetration). The excipients selected depend on the solubility of the API as well as the concentration needed to provide the necessary dose in each spray. The formulation needs to be made so it maximizes contact with the nasal mucosa, holds off clearance for as long as possible, and is rapidly absorbed.
While most nasal drugs are spray formulations, dry powders have some advantages. These include enhanced chemical stability, lower risk of microbial spoilage (which means fewer preservatives), and the ability to administer larger amounts of API in each dose.
There are many challenges to consider during the development of a nasal formulation, including:
- Overcoming poor solubility of the API
- Minimizing clearance
- Increasing bioavailability
- Ensuring compatibility with the delivery device
- Improving patient acceptance
Nasal Formulation Strategies
Solubility of API
The API needs to be formulated with excipients that enhance adhesion and, therefore, absorption in the nasal mucosa. Determining these excipients can be accomplished with customized solubility software that uses Hansen solubility parameters to empirically discover a vehicle that is compatible with nasal delivery and maximizes the solubility of the API.
Minimizing Clearance
The rapid removal of particles that land on the surface of the nasal cavity—normally in as little as 10–20 minutes—can limit the absorption and bioavailability of a nasal drug. Bioadhesives in a formulation can slow down this clearance increasing residence time to as much as 30 minutes.
The Importance of Bioavailability
Bioavailability is always an essential consideration during the development of a new nasal drug. Absorption via the nasal mucosa directly into the bloodstream bypasses the liver and first-pass metabolism, a key consideration for drugs that have poor oral bioavailability. The bioavailability of large-molecule drugs can be improved with permeation enhancers.
The bioavailability of nasally administered APIs can be compatible with that of injected drugs in many cases. For example, an intranasal formulation of diazepam was shown to have 96% absolute bioavailability compared to intravenous diazepam in a phase 1 crossover trial of healthy volunteers.2
Co-Development of Formulation and the Device
Successful development and launch of a nasal drug delivery system requires cooperation between teams that are involved in formulation and device development. This ensures that data generated during the drug development and testing phases will be relevant for manufacturing and product launch.
The intended deposit location in the nasal cavity is important for efficacy and will depend on both the formulation and the device. The delivery device, whether a spray pump or a meter-dose inhaler, must be effective for delivering a precise dose to this target area. The device should also be easy to use by the intended patient population.
Conclusion
Given the benefits of nasal drug delivery and the value of this segment of the industry, it is no wonder that the number of nasal drug NDAs are increasing. As pharmaceutical companies continue to explore this innovative technology, they will need to partner with a company that has extensive experience with a full range of formulation, analytic, and manufacturing services to develop complex nasal drug products and bring them to market.
At Particle Sciences, we offer a range of services to help advance complex nasal drug products including drug product development and manufacturing. If you want to know more about nasal delivery, contact us to discuss your project and see how Particle Sciences can take your product from concept through commercialization.
Authors: Robert Lee and Nick DiFranco

