A 2024 Guide to Solubility Improvement and Bioavailability Enhancement Techniques
Overcoming poor water solubility of active pharmaceutical ingredients (APIs) is an ongoing challenge for drug manufacturers. The issue affects an estimated 90% of new APIs, presenting a major hurdle in drug product development.
Common Solubility-Enhancing Methods
A common model for describing solubility and permeability of APIs is the Biopharmaceutical Classification System (BCS), which divides molecules into four quadrants based on their behavior in a pre-defined aqueous environment (Figure 1). APIs that fall under a BCS Class II designation suffer from low solubility but exhibit high tissue permeability—meaning they are ideal candidates for solubility enhancement. BCS Class IV molecules—which exhibit low solubility and low permeability—require a combination of solubility enhancement and permeation enhancement to achieve a therapeutic effect.
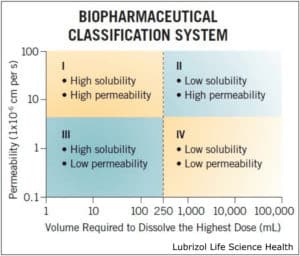
Figure 1: The biopharmaceutical classification system, which classifies APIs based on solubility and permeability.
Drug manufacturers can choose from several methods to increase the solubility and bioavailability of BCS Class II and IV APIs. These are commonly divided into four categories:
- API Physical Modification
- API Chemical Modification
- Encapsulation
- Inclusion Complexes
API Physical Modification
Nanomilling
Nanomilling is a process by which the particle size of an API is reduced in a liquid vehicle (typically aqueous) via grinding using polymeric or ceramic media. Nanomilled API particles exhibit increased dissolution rates due to their higher surface area-to-volume ratios, and the nanoparticulate suspensions may be formulated into stabilized liquids, lyophilized powders, solid dosage forms, or a variety of other form factors for multiple routes of administration.
Micronization
Like nanomilling, micronization increases solubility by increasing the surface area-to-volume ratio of API particles. Micronized API particles are typically generated through processes like jet milling, which uses pressurized air to reduce particle sizes to the micron scale.
Co-crystals
Co-crystals are engineered materials comprised of API molecules and co-crystal formers (AKA coformers) which come together to form a single crystal lattice. Ideally, this lattice will exhibit higher solubility than either individual component. Co-crystal formation can be achieved via multiple techniques and does not rely on ionic interactions, so it can be applied with non-ionizable APIs.
Amorphous solutions and dispersions
When APIs are in crystalline form, the first barrier to dissolution is disrupting the crystal lattice. In the case of non-crystalline, or amorphous, solutions and dispersions, the crystal lattice is already disrupted so the solubility may be significantly higher. These materials are commonly prepared via spray drying or hot melt extrusion processes using excipients that stabilize the API in an amorphous form.
API Chemical Modification
pH modification
The solubility of ionizable APIs can depend on the pH of their solution. pH is modified by adding acidic or basic excipients.
Salt formation
APIs are often more soluble in salt form. With this technique, an API is converted to salt by coupling it with a counterion and crystallization solvents. Salt formation is only possible for ionizable APIs, and the solubility of an API salt may be significantly impacted by the choice of counterion.
PEGylation
Polyethylene glycol (PEG or macrogol) is a non-toxic, non-immunogenic and highly water-soluble compound. Through PEGylation, hydrophilic PEG molecules are covalently or non-covalently attached to the surface of API particles, forming a conjugate. PEGylated API exhibits higher water solubility and a longer serum half-life, both of which contribute to greater efficacy of the API. The majority of FDA-approved PEGlyated drug products are biologics such as proteins, peptides, and oligonucleotides, however there are also PEGylated small molecules and liposomal drug products on the market today.
PEGPLUS™ technology
A multifunctional excipient technology offered by the Particle Sciences with many applications such as improving adhesion to mucous membranes (increasing bioavailability), coating surfaces to prevent unwanted adhesion of cells and bacteria, and protecting/stabilizing therapeutic agents in the body. PEGPLUS Technology has proven to be safe and effective in multiple formulations. Learn more about the technology here.
Encapsulation
As well as increasing solubility, encapsulation techniques with nano- and micro-scale carriers are used for protecting drugs from degradation in harsh environments, allowing sustained release over time, or targeted delivery.
Micelles
Surfactants (surface-active agents or SAAs) are amphiphilic compounds with a hydrophilic head and a hydrophobic tail. When surfactants aggregate, they form micelles, which are typically spherical structures (although other conformations are possible). The outer surface of the micelle consists of hydrophilic heads that face the surrounding aqueous environment, whereas the hydrophobic tails face inwards, forming a space where hydrophobic APIs are soluble.
Liposomes
Liposomes are similar to micelles in that they are spherical and formed from amphiphilic compounds with hydrophilic heads and hydrophobic tails called phospholipids. When liposomes are formed, the phospholipids arrange in one or more bilayer structures surrounding an aqueous core. Liposomes are used to encapsulate water-soluble APIs in the core, oil-soluble APIs in the bilayer membrane, and, if made of cationic phospholipids, trap and deliver oligonucleotides for gene therapy.
LyoCell® technology
A patented drug delivery technology based on the use of reverse cubic phase lyotropic liquid crystals. LyoCells possess powerful drug-solubilizing properties and are compatible with a variety of APIs, whether small molecule or biopharmaceutical. LyoCell technology can also instill better-controlled drug release compared to other technologies and can potentially handle a high payload of API. Learn more about the benefits of LyoCell technology here.
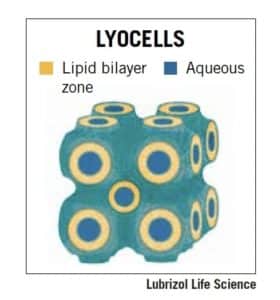
Figure 2: A graphical representation of LyoCell technology.
Solid-lipid nanoparticles
Solid-lipid nanoparticles (SLNs) are submicron particles of lipid material (usually phospholipids, triglycerides, and natural waxes). SLNs can be used as a delivery vehicle with a wide range of APIs—including both poorly water-soluble APIs and high molecular weight water soluble compounds such as proteins, peptides, and nucleic acids. Solid-lipid nanoparticles are relatively inexpensive and generally recognized as safe by the FDA.
Polymer encapsulation
Several FDA-approved drug products utilize polymer encapsulation to create long-acting dosage forms. Depot injections often contain bioresorbable microparticles comprised of polymer—typically poly-lactic-co-glycolic acid (PLGA)—and API. Through careful polymer selection and particle engineering, these dosage forms enable highly controlled, parenteral delivery of small molecule APIs or biologics which cannot be effectively delivered via traditional routes.
Inclusion Complexes
β-cyclodextrins
These cyclic oligosaccharides have a torus shape with a hydrophilic surface and a hollow hydrophobic core, where APIs can be entrapped via non-covalent interactions. Once the complex is formed, β-cyclodextrins act as water-soluble carriers for APIs with the ability to partition into biological barriers such as skin and mucus membranes to increase drug absorption.
Serum albumin
There are two types of serum albumin used in drug delivery—human serum albumin (HSA) and bovine serum albumin (BSA). Serum albumin is the most abundant protein in vertebrate blood and naturally acts as carrier of nutrients, making it a non-toxic, biocompatible material. As a solubility enhancement tool, serum albumin can be complexed with APIs to form bioresorbable nanoparticles with improved solubility and dissolution rate.
The Potential and Versatility of Nanomilling
In our experience, we’ve found nanomilling to be a particularly efficient, reproducible, and scalable process for formulating poorly water-soluble APIs. Nanomilling is used to increase the dissolution rate of poorly water-soluble APIs. This technique has been used in numerous FDA-approved drug products since the year 2000. As mentioned above, nanomilling is similar to micronization, whereby the particle size of an API is reduced through a mechanical process, increasing the surface-area-to-volume ratio and dissolution rate of API particles. Nanoparticulate suspensions generated via nanomilling have been converted into a wide range of dosage forms, including oral liquids, capsules, tablets, films, injectables, aerosols, and more.
Nanomilling is categorized as a “top-down” approach to solubility enhancement, wherein crystalline API structures are physically broken down into smaller nanoparticles. This differs from “bottom-up” methods such as liposomal and polymeric encapsulation, where particles are assembled from components. Another bottom-up approach is solvent/anti-solvent precipitation, which may lead to nanoparticles.
Advantages of nanomilling
Nanomilling has several advantages over other solubility enhancement methods:
- Nanomilling improves solubility without the addition of solvents, the use of pH extremes, or the inclusion of potentially toxic solubilizers or emulsifiers like Cremophor®.
- Well-formulated nanoparticulate suspensions can contain high API concentrations (anywhere from 5 to 40+% w/w) and may be more efficient than bottom-up solubility enhancement techniques.
- The nanomilling process is commercially scalable and reproducible, as evidenced by the 12+ FDA-approved nanomilled products.
Challenges of nanomilling
Despite its many advantages, nanomilling is not without its challenges:
- The increased surface area-to-volume ratio of particles also generates higher surface energy, which tends to make nanoparticulate suspensions unstable. Therefore, proper stabilizer selection is critical.
- Nanoparticulate suspensions can undergo a spontaneous process called Ostwald Ripening, wherein the mean particle size grows over time as small particles dissolve and redeposit onto larger ones. This effect can be counteracted with the right stabilizers in the right concentrations.
- Overmilling, or milling for extended periods, can also lead to stability challenges, so each API requires process development to determine the ideal processing time and conditions.
These formulation and process development challenges can be minimized with an experienced formulation team that understands how milling processes, stabilizer selection, and the physicochemical properties of an API contribute to successful product development.
Conclusion
Solving solubility and bioavailability challenges for BCS Class II and IV APIs is not a one-size-fits-all endeavor. Selecting the ideal technique or combination or techniques requires a thorough understanding of the target product profile, including a clear view of the intended dosage form and route of administration.
Finding a partner with an open mindset and experience across a range of bioavailability enhancement techniques increases your chances of success. At Particle Sciences, we offer pre-defined feasibility programs around nanomilling and polymer encapsulation, but we regularly employ the other techniques described in this post. Ultimately, our goal is to achieve your target product profile and help get your product to market. If you are facing solubility and bioavailability challenges, contact us to discuss your options and see how Particle Sciences can take your product from concept through commercialization.
Authors: