As a leading CDMO partner, we transform your discovery into a clinic-ready product.
Particle Sciences was sold by Lubrizol and acquired by Agno Pharmaceuticals, a Global Premier CDMO on December 15, 2023. Formerly known as Particle Sciences, Health – CDMO Division and founded in 1991, Particle Sciences has established itself as a leading contract development and manufacturing organization. Staffed by experienced CDMO industry experts, we offer complex drug product formulation development and manufacturing, drug-eluting device development and manufacturing, and a comprehensive suite of supporting services, including analytical and physical characterization and cGMP clinical supply manufacturing. We specialize in offering a variety of formulation development technologies, including the ability to deal with poorly soluble and highly potent compounds under GxPs in both sterile and non-sterile environments, which helps to distinguish us from other CDMO service providers.
Solutions
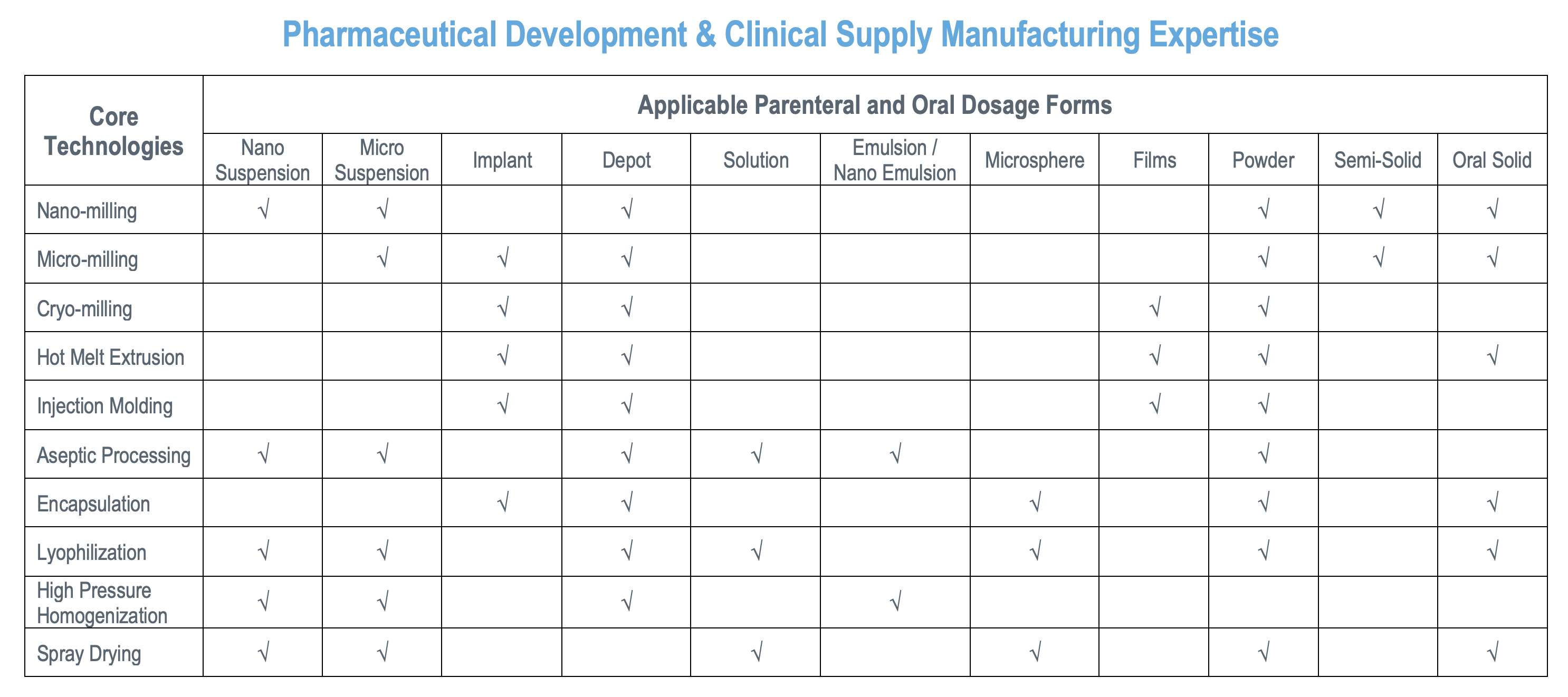
We have been providing an array of delivery solutions for poor bioavailability drugs and long-acting injectables & drug-eluting devices since 1991. Our multi-purpose facility is located in Bethlehem, PA, USA and is an US-FDA-approved, DEA-licensed (I-V) site that is capable of working with highly potent APIs and offers aseptic manufacturing.
Today, our Clients include over half of the leading pharmaceutical and biotech companies, foundations, universities, and privately-backed start-ups.
About Agno Pharmaceuticals
Agno Pharmaceuticals is a global small molecule CDMO supplying critical RSMs, intermediates, active pharmaceutical ingredients (APIs), highly potent APIs, sterile APIs, and sterile drug product formulations to large and medium-sized pharmaceutical, biotech, generic and CDMO customers. Founded in 2004, Agno has process development and US-FDA inspected GMP-compliant manufacturing facilities with sterile suspension injectable manufacturing capabilities. In addition, Agno has established a drug product formulation research and development laboratory for complex injectables. Agno Pharmaceuticals has invested in its people and facilities and established a broad spectrum of service capabilities throughout the entire drug development and commercialization process. Agno Pharmaceuticals has an excellent track record in the delivery of both development and commercial stage cGMP manufacturing solutions to its customers in North America and Europe.
Visit Agno Pharma.
